
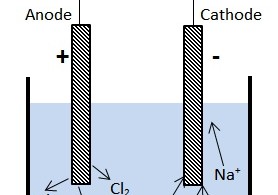
The H a further involves in electrochemical desorption (Heyrovsky reaction) or chemical desorption (Tafel reaction) to create hydrogen gas or interacts with the reducible chemicals like chlorinated substances, which leads to HDC. The water electrolysis at the cathode leads to the hydrogen evolution via electrochemical hydrogen adsorption (Volmer reaction) where atomic hydrogen (H a) is chemically adsorbed on active site of the electrode surface (M). Electrochemical reduction through hydrodechlorination (HDC) occurs at the cathode due to water electrolysis.
Recently, electrochemically-induced reduction has gained interest for removal of COCs from groundwater ( Li and Farrell, 2000 Petersen et al., 2007b Mishra et al., 2008 Mao et al., 2011, 2012a, 2012b).

So far, the efficient reductive dechlorination of chlorinated organic compounds (COCs) was achieved via zero-valent iron and palladium-based materials ( Lowry and Reinhard, 2001 Lien and Zhang, 2005 Liu et al., 2006 Wu and Ritchie, 2006 Petersen et al., 2007a Phillips et al., 2010 Ma et al., 2012 He et al., 2013). The presence of chlorine in the TCE structure contributes to its carcinogenic and mutagenic properties thus, complete dechlorination is favorable for degradation of TCE dissolved in groundwater. The USEPA has set Maximum Contaminant Levels (MCLs) for TCE in drinking water at very low concentrations of 5 μg L −1. Improper disposal of TCE coupled with its physical and chemical properties (low solubility and limited degradation) led to persistent TCE contamination at many hazardous waste sites ( Moran et al., 2007). Trichloroethylene (TCE) has been widely used as an ingredient in industrial cleaning solutions and as a “universal” degreasing agent. The results indicate that the cathode material affects TCE removal rate while the Pd catalyst significantly enhances cathode activity to degrade TCE via HDC. TCE removal decreased from 99% to 41.2% in presence of 100 mg L −1 of nitrates due to the competition with TCE for HDC at the cathode. The performance of the palladized Fe foam cathode was tested for degradation of TCE in the presence of nitrates, as another commonly found groundwater species. With a higher electrocatalytic activity than Ni, Pd catalyst (used as cathode coating) increased TCE removal from 43.5% to 99.8% for Fe, from 56.2% to 79.6% for Cu, from 68.4% to 78.4% for Ni, from 42.0% to 63.6% for Al and from 64.9% to 86.2% for C cathodes. Different performances of the cathode materials originate from differences in the bond strength between atomic hydrogen and the material. Ni foam cathode produced the highest TCE removal (68.4%) due to its high electrocatalytic activity for hydrogen generation and promotion of HDC. We tested commercially available foam materials, which provide important electrode surface are and properties for field application of the technology. The performance of iron (Fe), copper (Cu), nickel (Ni), aluminum (Al) and carbon (C) foam cathodes was evaluated. A cathode followed by an anode electrode sequence was used to support reduction of TCE at the cathode via hydrodechlorination (HDC). It is often the underlying metal substrate.In this study, different cathode materials were evaluated for electrochemical degradation of aqueous phase trichloroethylene (TCE).


 0 kommentar(er)
0 kommentar(er)
